June 15, 2020
A recently published study explored how an unconventional treatment method undergoing clinical trials in cancer patients could help lessen the severity of lupus nephritis (inflammation of the kidneys), a common and serious condition in people with lupus. Funded by the Lupus Research Alliance, this study, published in the journal, Science Translational Medicine, was led by graduate student Dr. Ping-Min Chen and Dr. Joseph Craft at Yale University. They took a deeper look at how signaling between T cells and antibody-secreting cells contribute to this kidney condition, and how these immune cells could be targeted to help reverse the effects they create.
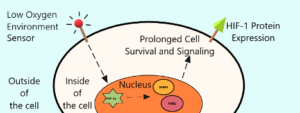
Dr. Craft, Dr. Chen and their team carried out this study with mice that were subject to developing lupus, and as a result, lupus nephritis. Some organs, such as the kidneys, contain very hypoxic regions, meaning areas with low levels of oxygen. This low-oxygen environment is worse in lupus patients due to their underlying inflammation. The damage to kidney tissue is triggered by the destructive, autoreactive antibodies of lupus patients, released by antibody-secreting cells. Immune cells typically use oxygen to function, but hypoxic environments can cause immune cells to display a protein within the cells called hypoxia-inducible factor-1 (HIF-1), which allows them to survive and function for longer periods of time, leading to more inflammation and greater kidney damage.
These scientists were then able to remove specific immune cells, T cells, which had built up in the kidneys of these mice, and using complex lab technology called RNA sequencing, they created a detailed genetic profile of how these T cells were programmed to work. This profile served as a map for the many different genes and proteins displayed on the inside and outside of these T cells, respectively. The lab confirmed that there were large amounts of HIF-1 in these T cells, allowing them to survive in a low-oxygen environment.
To control the amount of HIF-1 on the body’s immune cells, a drug called PX-478 which has been tested as a possible cancer treatment, was given to lupus-prone mice. After several weeks, the scientists observed that the mice that received the treatment displayed significantly less kidney damage and lower numbers of T cells.
Excited by these results, the group looked specifically at the impact of having T cells with HIF-1 in the kidney. They studied mice that could develop lupus but did not express HIF-1 in their T cells even when oxygen levels were low. These mice showed less kidney damage than those lupus-prone mice that did express HIF-1. Finally, to confirm these observations in humans, a collaboration with Dr. Marcus Clark at the University of Chicago studied tissue samples taken from lupus patients with nephritis and stained with a dye that recognizes HIF-1 and other hypoxia-induced proteins. Just like the lupus-prone mice, the kidney tissue samples showed high levels of these proteins in T cells.
Investigators concluded that more studies are needed to confirm the safety profile of this potential lupus treatment. They are concerned that stunting the immune system by blocking HIF-1 could open the door to unwanted uncontrolled bacterial or viral infections. However, as they report, their early findings show that PX-478 shows promise as a potential therapy for lupus nephritis because of its ability to limit the activity of damage-inducing T cells in the kidney while decreasing the number of autoreactive antibodies produced, and successfully lessening kidney damage.
Caption: When T cells enter a hypoxic (low oxygen) environment, like the kidneys, sensors on their surface send a signal to the brain of their cell called the nucleus, which commands its function. Inside the nucleus, genes are activated for the expression of specific molecules called transcription factors which allow for the creation of specific proteins by the cell. In this case, those transcription factors are HIF-1a, BNIP3, and PDK2, which all become activated by the hypoxic environment. As a result of these activities, the cell makes and expresses a protein on its surface called HIF-1, which allows the cell to live longer and remain activated, which supports inflammation. The goal of the study mentioned in this article is to inhibit expression of HIF-1 on T cells to lessen the inflammatory signaling in the kidneys.